Pregnancy is a known risk factor for thrombosis or blood clots in the leg veins (deep vein thrombosis) and lungs (pulmonary embolism). Injectable blood thinners called low-molecular-weight heparin (LMWH) may be prescribed during pregnancy to prevent or treat blood clots. This may include people who have had a new or prior blood clot or those who have strong risk factors for a blood clot.
This can create a challenge around labor and delivery, because staying on blood thinners can increase the risk of bleeding and affect the ability to get an epidural or spinal anesthetic for pain control. There are different approaches around the world for managing blood thinners around labor and delivery. Generally, blood thinners are stopped temporarily before delivery to prevent bleeding and are restarted after delivery to prevent blood clots in the postpartum period. Different doses of blood thinners may be used in different scenarios or hospitals. Sometimes, a planned delivery like an induction of labor is recommended.
The safest time to stop and restart the blood thinners is unclear and may influence being able to have a spontaneous delivery or a planned delivery like an induction of labor.
Our international research study will help give doctors and future pregnant patients more information about managing blood thinners around delivery and the postpartum period.
Individuals who are on injectable blood thinners during pregnancy and postpartum because of a risk of blood clots.
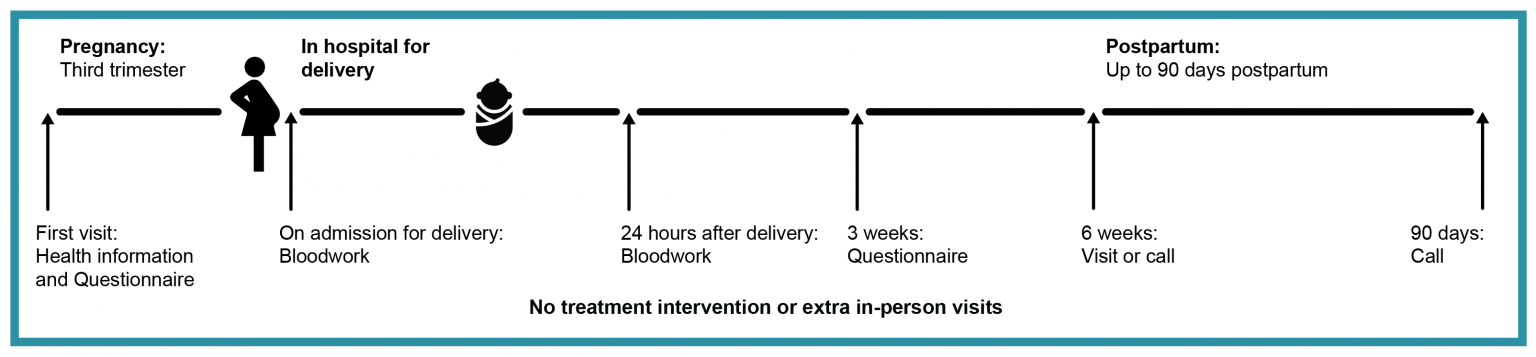
This is an observational study, which means that there is no treatment intervention.
We will first approach an individual during pregnancy to see if they are interested in the study. There will be one research visit during pregnancy to collect information about their health, pregnancy, and blood thinners. We will try and do this at the same time as a regular clinical visit when possible. There may be the option of doing this by phone or on a virtual platform. We will also ask the participant to complete a short online or paper questionnaire to better understand their perspective about their upcoming delivery.
We will collect information about the delivery, and their blood thinners around labor and birth. There will be blood work when they are admitted to the hospital and the day after delivery, if it is not already done for usual care.
At around 3 weeks postpartum, participants will complete a second short online questionnaire. At 6 weeks and 90 days postpartum, we will have a short follow-up visit to ask questions about their delivery, blood clot and bleeding symptoms. These research visits can be over the phone, or a virtual platform if available.
Please visit our ‘About Us – The Team’ page to learn what locations are participating around the world.